Bone Metastasis Risk Calculator
This calculator estimates your risk level for bone metastasis based on primary cancer type and clinical factors. Results provide general guidance only and should not replace professional medical advice.
Enter your details and click Calculate to see your risk assessment.
Key Takeaways
- When cancer cells invade bone, they hijack normal remodeling, leading to bone loss and higher fracture risk.
- Breast, prostate, lung, and kidney cancers are the top culprits for bone metastasis.
- Osteoclast over‑activity and osteoblast suppression drive the destructive process called osteolysis.
- Early imaging, blood markers, and bone‑strength assessments can catch problems before a break happens.
- Treatment combines systemic cancer therapy with bone‑protective drugs, physical activity, and nutrition.
Understanding the link between Tumor Growth the uncontrolled multiplication of malignant cells within a tissue and Bone Health the strength, density, and structural integrity of the skeletal system is crucial for anyone dealing with cancer. Tumors don’t stay put; they can spread, or metastasize, to bone, where they rewrite the normal remodeling script. This article walks through why that happens, what it looks like clinically, and how you can protect your skeleton while fighting cancer.
How Tumors Interact with Bone Tissue
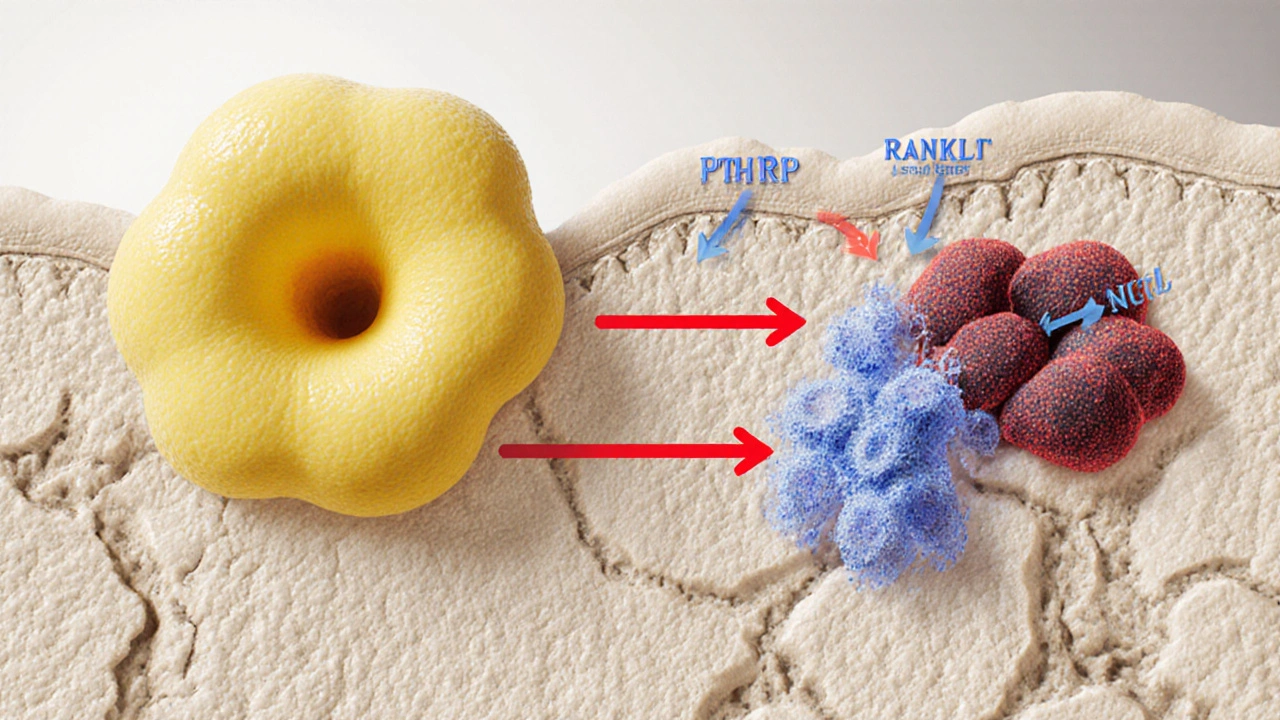
Bone isn’t a static scaffold; it constantly renews itself through a balance between Osteoclasts cells that break down bone tissue and Osteoblasts cells that build new bone matrix. When cancer cells lodge in bone, they secrete factors-like parathyroid hormone‑related protein (PTHrP), interleukins, and prostaglandins-that tip the scale toward osteoclast activation. At the same time, they release signals that mute osteoblast activity. The outcome is a net loss of bone mass, a process clinicians call osteolysis.
This imbalance creates weak spots that can fracture under normal stress. It also releases growth‑promoting calcium and growth factors back into the tumor, creating a vicious feedback loop.
Common Cancers That Spread to Bone
Not every cancer loves bone. Epidemiological data show that about 70% of bone metastases originate from four primary sites:
- Breast cancer: tends to cause mixed osteolytic and osteoblastic lesions.
- Prostate cancer: often leads to bone‑forming (osteoblastic) lesions, but the new bone is brittle.
- Lung cancer: usually produces purely osteolytic destruction.
- Kidney (renal) cancer: creates aggressive osteolytic spots.
These cancers differ in the molecules they release, which explains why some cause mostly bone loss while others lead to abnormal bone formation.
Biological Mechanisms: Osteoclast Activation and Osteoblast Suppression
The key pathways include:
- RANK/RANKL/OPG axis: Tumor cells boost RANKL (receptor activator of nuclear factor κB ligand) and lower OPG (osteoprotegerin), a natural decoy. More RANKL means more osteoclasts.
- Growth factors: TGF‑β, IGF‑1, and BMPs released from bone matrix during resorption feed the tumor.
- Inflammatory cytokines: IL‑1, IL‑6, and TNF‑α further stoke osteoclast activity.
When osteoblasts are suppressed, the new bone that forms is disorganized and weak, even in cancers that appear “osteoblastic.” This explains why prostate cancer patients still suffer fractures.
Clinical Signs and Diagnosis
Patients often report new bone pain, especially at night or with weight‑bearing. Sometimes a pathological fracture-one that occurs with minimal trauma-is the first clue.
Diagnostic tools include:
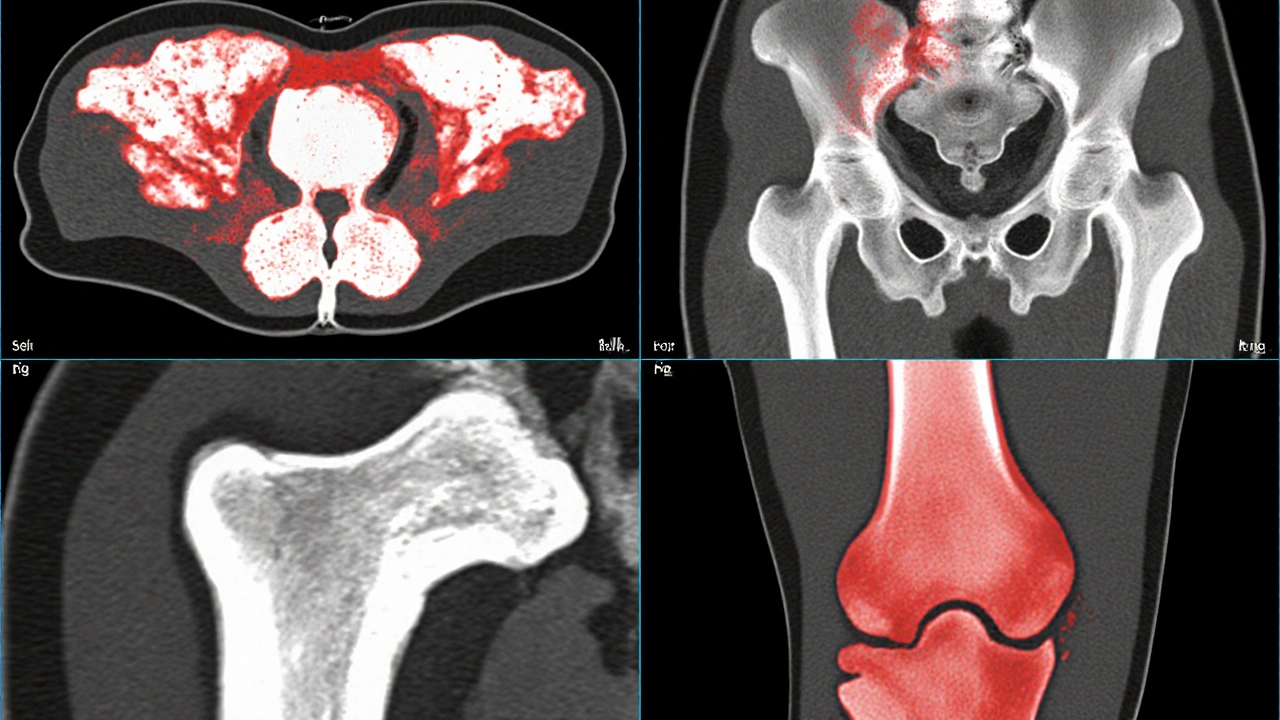
- Imaging: X‑ray, CT, MRI, and bone scintigraphy. PET/CT with ^18F‑FDG is increasingly used for whole‑body staging.
- Blood markers: Elevated alkaline phosphatase suggests increased bone turnover; serum calcium may rise in osteolytic disease.
- Biopsy: Confirms that a bone lesion is metastatic rather than a primary bone tumor.
Early detection lets clinicians start bone‑protective measures before a fracture occurs.
Impact on Bone Strength and Fracture Risk
Bone mineral density (BMD) drops dramatically in metastatic zones. Studies measuring BMD by DXA in breast cancer patients with bone mets show a 30‑40% reduction compared with age‑matched controls.
Fracture risk isn’t just about density; it’s also about architecture. Micro‑CT analyses reveal that metastatic bone has thinner trabeculae and larger cortical pores, both of which undermine mechanical strength.
Consequences include:
- Pathological fractures of the femur, spine, or pelvis-often requiring surgery.
- Spinal cord compression from vertebral collapse, a medical emergency.
- Reduced mobility, leading to secondary complications like deep‑vein thrombosis and loss of independence.

Treatment Strategies to Protect Bone Health
Managing bone complications involves a two‑pronged approach: controlling the primary tumor and directly protecting bone.
- Systemic cancer therapy: Chemotherapy, hormonal therapy, targeted agents, and immunotherapy shrink the tumor load, indirectly reducing bone‑derived signals.
- Bone‑targeted drugs:
- Bisphosphonates drugs that bind to bone mineral and inhibit osteoclast-mediated resorption (e.g., zoledronic acid) are first‑line for most solid‑tumor bone mets.
- Denosumab a monoclonal antibody that blocks RANKL, preventing osteoclast formation is an alternative, especially when renal function limits bisphosphonates.
- Radiotherapy: Localized radiation can relieve pain and strengthen bone by reducing tumor burden.
- Surgical stabilization: For impending or actual fractures, orthopedic fixation restores function.
Clinical guidelines recommend initiating bone‑protective agents at the time of diagnosis of bone metastasis, not waiting for a fracture.
Lifestyle Tips for Patients
Even while undergoing aggressive therapy, everyday habits can help maintain bone strength:
- Calcium and Vitamin D: Aim for 1,200mg calcium and 800‑1,000IU vitamin D daily, unless contraindicated.
- Weight‑bearing exercise: Short walks, resistance bands, or low‑impact aerobics stimulate osteoblast activity.
- Avoid smoking and excess alcohol: Both accelerate bone loss.
- Fall‑prevention: Keep walkways clear, use handrails, and wear supportive shoes.
These steps don’t replace medical treatment but can reduce the chance of a break.
Future Directions
Research is racing to find smarter ways to break the tumor‑bone feedback loop. Emerging therapies include:
- RANKL inhibitors with dual action: Next‑generation antibodies that also block tumor‑derived growth factors.
- Bone‑targeted radioisotopes: Such as radium‑223, which delivers radiation directly to bone lesions while sparing soft tissue.
- Gene‑editing approaches: CRISPR‑based strategies aim to silence osteoclast‑activating genes within the tumor microenvironment.
While these are still in trials, they hint at a future where bone health is preserved without compromising cancer control.
Frequently Asked Questions
Can bone‑protective drugs prevent fractures?
Yes. Bisphosphonates and denosumab have been shown in multiple trials to reduce skeletal‑related events by 30‑40% in patients with bone metastases.
Is pain the first sign of bone involvement?
Pain is the most common early symptom, especially deep, constant pain that worsens at night. However, some patients develop fractures before noticeable pain.
Do all cancers spread to bone?
No. Hematologic cancers like leukemia can involve bone marrow, but solid tumors such as colorectal or pancreatic cancer less frequently seed bone.
How often should I get bone scans?
Guidelines suggest a baseline scan at diagnosis of metastasis, then every 3-6months or sooner if new symptoms appear.
Can exercise really help when I have bone metastases?
Gentle, weight‑bearing activity can stimulate bone formation and improve balance, lowering fall risk. Always get clearance from your oncologist or physiatrist first.
Bottom line: tumor growth that reaches bone isn’t just a side effect-it fundamentally reshapes the skeleton, raising fracture risk and hurting quality of life. By recognizing the warning signs, using imaging wisely, and starting bone‑protective therapy early, patients can keep their bones stronger while fighting the underlying cancer.



Wow, this whole bone metastasis calculator feels like a half‑baked attempt at science journalism, and yet somehow it’s being paraded around as if it were a breakthrough. The UI is clunky, the language is over‑inflated, and the underlying assumptions are, frankly, dangerously simplistic. First, presenting a "risk level" without any discussion of confidence intervals or statistical validation is academically irresponsible. Second, the weighting scheme for tumor size and lesion count seems arbitrarily assigned rather than grounded in peer‑reviewed data. Third, the emphasis on pain scores ignores the fact that many patients experience silent progression until imaging reveals catastrophic damage. Fourth, the lack of disclaimer about the heterogeneity of cancer subtypes is a glaring omission that could mislead laypeople. Fifth, even the color coding-low risk in soothing greens and high risk in alarming reds-plays into cognitive bias rather than fostering informed decision‑making. Sixth, the tool fails to address the role of systemic therapies that can alter bone remodeling dynamics. Seventh, there is no integration with electronic health records, making it a siloed, useless gimmick for clinicians. Eighth, the text copy is peppered with typos like "definately" that betray a sloppy editorial process. Ninth, the reliance on self‑reported pain scores assumes patients will be honest and precise, an assumption proven false in countless studies. Tenth, the calculator does not account for comorbidities such as osteoporosis that dramatically influence fracture risk. Eleventh, the absence of a citation list leaves readers with no way to verify claims. Twelfth, the script’s JavaScript validation is rudimentary, potentially allowing nonsensical inputs to slip through. Thirteenth, there is an implicit suggestion that a higher score equals a higher need for aggressive treatment, which is a dangerous oversimplification. Fourteenth, the overall tone feels alarmist, possibly scaring patients who could otherwise benefit from nuanced clinical guidance. Fifteenth, the tool lacks accessibility features for visually impaired users, which is a basic compliance issue. Sixteenth, the content could have been enriched with patient stories to humanize the data, but instead it feels sterile and detached. In short, this calculator is a superficial veneer that does not stand up to rigorous scrutiny, and it would be better off hidden until a proper evidence‑based overhaul is performed.
I think the interactive approach is a nice way to engage people who might otherwise ignore the stats. It makes the data feel personal, which can be a good motivator for folks to discuss their situation with a doctor.
It’s also helpful that the tool doesn’t pretend to replace professional advice.
From a clinical perspective, the inclusion of oncogenic signaling pathways and osteolytic lesion quantification is crucial. However, the current algorithm appears to weight tumor size linearly, which neglects the nonlinear dynamics of bone resorption mediated by RANKL‑expressing tumor cells. Incorporating a log‑scale adjustment for lesion count could improve predictive fidelity.
Honestly, it’s a moral failing that such a superficial tool is out there. If patients act on it without proper context, they could be misled into unnecessary panic or false reassurance. The creators should take responsibility and seek proper medical endorsement.
Well, isn’t this just the perfect example of modern techno‑optimism gone awry? They give you a shiny calculator, but where’s the nuance? You get a score and a color, and suddenly you’re supposed to trust it as if it’s a crystal ball.
Meanwhile, the real world is messy-tumors adapt, bones remodel, pain perception varies, and yet this thing pretends to capture it all in a few boxes.
It’s almost comedic, the way it reduces complex pathophysiology to a ‘moderate‑risk’ badge. If you’re looking for a quick thrill, sure-press the button. If you actually care about your health, you’ll consult an oncologist and a orthopedic specialist, not a web widget.
In short, it’s a glorified quiz, and the sarcasm is that people might treat it like serious medical guidance.
Looks fine.
This feels like an over‑dramatized attempt at science-maybe it’s meant to grab attention, but the execution is half‑hearted. I’m not saying it’s bad, just that it could do with a stronger narrative to really hit home.
Hey folks, great job on making something people can actually use. Just remember it’s a starting point, not a definitive answer. Keep the optimism flowing!
From a methodological standpoint, the risk calculator lacks a transparent validation cohort and fails to disclose confidence intervals. Moreover, the scoring rubric appears ad‑hoc, which undermines its scientific credibility.
Hey there! I love how this tool tries to bring awareness to bone health in cancer patients. Just a gentle reminder: always double‑check with your healthcare team. Stay strong and keep pushing forward!
Honestly, I think most of this is just hype. You can’t boil down cancer progression to a few numbers. The real world is far more complicated than this simplistic model suggests.
🚀 Let’s keep the conversation alive! This calculator can be a stepping stone-use it, share your experience, and empower others. Together we can make a difference! 💪
While I appreciate the effort, I can’t help but notice the lack of cultural context. Different populations respond uniquely to bone metastasis, and this tool seems to ignore that vital aspect.
Great initiative! A collaborative approach between oncologists and orthopedists could refine these risk scores. Looking forward to seeing more data-driven updates.
In so far as this calculator purports to serve as a clinical adjunct, it is fundamentally deficient. The dearth of peer‑reviewed validation renders it, at best, an anecdotal contrivance.
I appreciate the effort, but I wonder about the tool’s adaptability across diverse clinical settings. It would be helpful to see how it integrates with existing patient management workflows.