
When you write a prescription, are you writing the brand name or the generic name? It might seem like a small detail, but this choice affects patient safety, healthcare costs, and even how well someone sticks to their treatment. The truth is, generic prescribing isn’t just a cost-saving trick-it’s the standard of care in most cases, backed by decades of evidence and global health guidelines.
What Generic Prescribing Actually Means
Generic prescribing means writing the International Non-proprietary Name (INN) of a drug-the active ingredient-instead of the brand name. So instead of writing "Lipitor," you write "atorvastatin." Instead of "Losec," you write "omeprazole." This isn’t about substituting a different drug. It’s about naming the exact same chemical that’s been proven to work.
The World Health Organization started the INN program in 1950 to bring clarity to drug names across languages and countries. Today, 193 nations use these standardized names. In the U.S., the FDA’s Orange Book lists which generic versions are therapeutically equivalent to brand drugs. In Europe, the EMA requires generics to prove they deliver the same amount of active ingredient into the bloodstream within a tight range (80-125% bioequivalence). That’s not a guess-it’s science.
And here’s the kicker: generics aren’t cheaper because they’re worse. They’re cheaper because they don’t carry the marketing, advertising, and patent protection costs of brand drugs. A generic atorvastatin tablet costs about £2.50 a month. The brand version? Around £30. Omeprazole? £1.80 vs. £15. Those numbers add up fast across millions of prescriptions.
Why Clinicians Should Prescribe Generics by Default
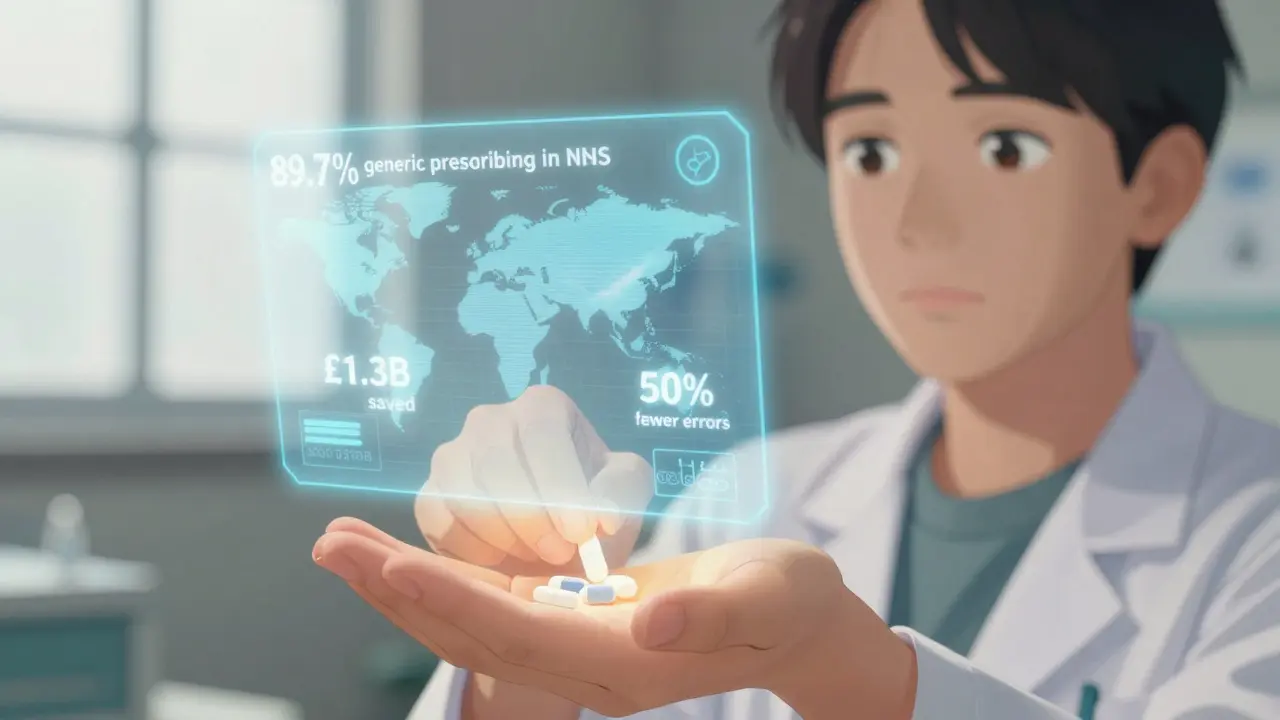
Every major medical body now recommends generic prescribing as the default. The American College of Physicians said in 2016 that prescribing generics improves adherence and outcomes while cutting costs. NHS England, which tracks every prescription in the country, found that 89.7% of prescriptions in 2022 were written generically-up from 86.2% in 2016. Primary care doctors hit 92.3%.
Why does this matter? Three big reasons:
- Cost savings for patients and systems-Generic drugs cost 80-85% less. In the NHS, generics make up 89.7% of prescription items but only 26% of total drug spending. That’s over £1.3 billion saved annually in England alone.
- Reduced medication errors-Imagine a patient gets prescribed "Zoloft," "Sertraline HCl," "Sertraline Hydrochloride," and "Sertraline" across different clinics. That’s four different names for the same drug. With generics, there’s only one name: sertraline. The Institute for Safe Medication Practices found this cuts errors by 50%.
- Better adherence-When patients pay less, they take their meds. A JAMA study showed that switching to generics led to 15% fewer hospitalizations for chronic conditions like hypertension and diabetes. The American College of Physicians estimates this could save the U.S. healthcare system $158 billion a year.
It’s not theoretical. Real patients benefit. A 2021 study of 3,200 patients found that when doctors explained why they were prescribing a generic, patient acceptance jumped from 67% to 89%. The biggest barrier? Misconceptions. Some patients think "generic" means "weaker." But the FDA and EMA require generics to meet the same purity, potency, and quality standards as brands. The only difference? The inactive ingredients-fillers, dyes, coatings-which rarely affect how the drug works.
When You Should NOT Prescribe Generically
There are exceptions. And they’re not random. They’re based on real clinical risks.
The British National Formulary (BNF) clearly defines three categories where brand-name prescribing is recommended:
- Category 1: Narrow therapeutic index drugs-These are medications where even tiny changes in blood levels can cause harm. Examples: warfarin (blood thinner), levothyroxine (thyroid hormone), phenytoin and carbamazepine (anti-seizure), and digoxin (heart medication). For these, switching between brands-even generic versions from different manufacturers-can cause INR fluctuations or seizure breakthroughs. The Epilepsia meta-analysis found a 1.5-2.3% increase in seizures with unmonitored switches.
- Category 2: Modified-release formulations-Drugs like theophylline or certain extended-release versions of antidepressants or pain meds. Their delivery systems are complex. A small change in how the drug is released can alter effectiveness. Pharmacists report 41% of them struggle with generic substitution here because release profiles don’t always match.
- Category 3: Biologics and biosimilars-Insulin, rheumatoid arthritis drugs like adalimumab, cancer treatments like rituximab. These are made from living cells, not chemicals. Even small manufacturing differences can trigger immune responses. The MHRA and FDA both require these to be prescribed by brand name to prevent automatic substitution. Biosimilars are not generics-they’re highly similar, but not identical.
That’s about 2% of all prescriptions. The rest? Generic is safe, effective, and recommended.

How to Implement Generic Prescribing in Practice
Switching your prescribing habits doesn’t have to be hard. NHS England’s Generic Prescribing Toolkit gives a clear 4-step plan:
- Audit your prescribing-Use tools like the Prescribing Analytics Dashboard to see what percentage of your scripts are generic. Most practices start at 70-80%.
- Learn the exceptions-Keep the BNF’s three-category list handy. Print it. Put it on your computer desktop. Memorize the top 10 high-risk drugs.
- Set defaults in your e-prescribing system-Most systems let you set generic as the default. Turn it on. If a patient needs a brand, you’ll have to override it intentionally. That’s the point.
- Monitor and adjust-Check your rates monthly. The NHS reports practices that follow this process hit 92-95% generic prescribing within six months.
And don’t forget the communication piece. Patients worry. They’ve been told for years that "brand is better." Use simple, clear language:
"This generic version has the same active ingredient as your old medication. It’s been tested to work the same way. It’s cheaper-about £12 a month less-and just as safe and effective. Many patients switch without any issues."
That script works. It reduces the nocebo effect-the phenomenon where expecting a drug to fail makes it feel like it does. One study found 30% of reported side effects or lack of effectiveness were due to patient belief, not the drug itself.
What’s Changing in 2025?
Guidelines aren’t static. In 2023, the MHRA added complex generics like glatiramer acetate (used for multiple sclerosis) to the brand-prescribing list because manufacturing variations affect how the drug behaves in the body. The FDA’s GDUFA III program now requires manufacturers to report adverse events linked to formulation differences-not just active ingredient issues.
And the future? Intelligent substitution. Instead of one-size-fits-all, we’re moving toward personalized decisions. For example: a patient on a stable dose of levothyroxine for five years? Keep them on the same brand. A new patient starting on atorvastatin? Prescribe the generic. Real-world data will help guide those choices.
By 2025, 75% of small-molecule drugs will have generic versions. But biologics? Only 40% will have biosimilars. So the need for clear, evidence-based prescribing rules will only grow.
What Patients Are Saying
Surveys show 82% of NHS patients are satisfied with generics. But 18% report concerns-mostly around sertraline and levothyroxine. That’s not because generics are inferior. It’s because these drugs are sensitive. Small changes in absorption can feel like a relapse. That’s why monitoring matters. A patient switching from branded to generic levothyroxine should have a TSH check in 6-8 weeks.
On Reddit’s r/medicine, a family doctor with 12 years of experience wrote: "95% of my patients do fine with generics. The 5% who have issues? Usually on antiepileptics or thyroid meds. I don’t switch them unless they ask-and then I monitor closely."
That’s the balance. Trust the data. Respect the exceptions. Communicate clearly.
Are generic drugs really as effective as brand-name drugs?
Yes. Generic drugs must meet the same strict standards as brand-name drugs for active ingredient, strength, dosage form, and route of administration. Regulatory agencies like the FDA and EMA require bioequivalence testing-proving the generic delivers the same amount of drug into the bloodstream within a narrow range (80-125%). Thousands of studies confirm they work the same way. The only differences are in inactive ingredients, which rarely affect how the drug works.
Why do some doctors still prescribe brand-name drugs?
There are three valid reasons: 1) The drug has a narrow therapeutic index (like warfarin or levothyroxine), where small changes in blood levels can be dangerous; 2) It’s a modified-release formulation (like extended-release theophylline), where the delivery system matters; or 3) It’s a biologic (like Humira or Enbrel), where manufacturing differences can trigger immune reactions. Outside these cases, prescribing generics is the standard of care.
Can I switch a patient from a brand to a generic safely?
For most drugs, yes-safely and routinely. For drugs with narrow therapeutic indices or complex delivery systems, it’s possible but requires caution. Always check the BNF or FDA Orange Book for exceptions. When switching, monitor the patient closely. For example, check INR levels within 2-4 weeks after switching warfarin, or TSH levels 6-8 weeks after switching levothyroxine. For most patients, no change is needed.
Why are biosimilars prescribed by brand name?
Biosimilars are not generics. They’re made from living cells, not chemicals, so tiny differences in manufacturing can affect how the body responds. Unlike small-molecule generics, biosimilars aren’t considered interchangeable by default. Prescribing by brand name ensures the same product is dispensed each time, reducing the risk of immune reactions or loss of effectiveness. The MHRA and FDA both require this.
Do generic drugs cause more side effects?
No. Large-scale studies and post-market surveillance show no increase in side effects with generic drugs. Some patients report feeling different after switching-but this is often due to the nocebo effect, not the drug itself. When patients are told generics are inferior, they’re more likely to notice or report side effects. Clear communication reduces this effect significantly.
Final Thought
Generic prescribing isn’t about cutting corners. It’s about cutting waste. It’s about giving patients the same medicine at a fair price. It’s about trusting science over marketing. And when you do it right-with clear exceptions, careful monitoring, and honest communication-you don’t just save money. You improve care.



Man, I remember when my grandma got switched from her brand-name thyroid med to generic. She swore up and down it wasn't working anymore. Turned out she just missed the fancy packaging. We sat down, talked it through, and she realized she wasn't actually feeling different-just scared. Turns out, a lot of us are scared of things that don't come in shiny boxes.
Generic doesn't mean cheap. It means smart. It means someone in Lagos or Lima can get the same medicine as someone in Manhattan. That's not just healthcare-that's dignity.
Been prescribing generics for 18 years. Never had a patient die because of it. Had a few who thought they were getting "second-rate" stuff. Took me five minutes of explaining bioequivalence and now they're the ones telling their friends to stop paying extra for logo pills.
My favorite line? "It's the same medicine, just without the ad budget."
My aunt switched to generic sertraline and said she felt "off" for two weeks. We checked with her doc-TSH, blood levels, everything fine. She just needed time to adjust her expectations. 🤷♀️
Also, why is it that the people who scream "brand is better" are usually the ones who can afford it? Just sayin'. 🤔
I think people get scared when they don't understand the science. Not because generics are bad but because they've been sold a story that brand equals better. It's not true. Just sayin'
generic is fine but i read somewhere that the fillers can cause allergies? like if you're allergic to corn or something? i dunno just wondering
Dear colleagues, I write this with the solemnity of a physician who has witnessed the devastation wrought by pharmaceutical greed. The fact that we must even debate the merits of generic prescribing is a moral indictment of our system. The WHO established INNs for equity-not profit. To prescribe brand names without clinical justification is to place corporate logos above human life. I implore you: audit your scripts today. Let not the almighty dollar dictate the dignity of a patient’s cure.
People who push generics are either broke or don't care about real medicine. If you're okay with a pill that's made in a factory where they don't even wash their hands, go ahead. I'll stick with the brand that actually works. This isn't about money. It's about not being a guinea pig.
So if I'm on levothyroxine and my doc switches me to generic, should I get my TSH checked right away? Just curious, because my cousin said she felt weird after switching.
Love this post! 🙌
Just last week I switched my dad from branded atorvastatin to generic. He was nervous, so I printed out the FDA bioequivalence chart and showed him. He laughed and said, "So I'm just paying for the color of the pill?"
Now he's saving $200 a year and says he feels the same. Win-win. 🙏
I used to think generics were sketchy. Then I got on a generic blood pressure med after losing my insurance. I didn't feel any different. My BP stayed the same. I didn't die. I'm still here.
Turns out, the drug companies didn't have magic in their pills. Just marketing.
GENERIC DRUGS ARE A GOVERNMENT PLOT TO CONTROL US. THE PHARMA COMPANIES ARE IN BED WITH THE FDA. THEY WANT YOU TO TAKE CHEAP PILLS SO YOU'LL BE TOO DULL TO REBEL. I READ ON A FORUM THAT THE FILLERS IN GENERIC ANTIDEPRESSANTS ARE LACE WITH LITHIUM TO MAKE PEOPLE CALM. I'M NOT KIDDING. LOOK IT UP.
Wow. You really believe this stuff? People who take generics are just dumb peasants who don't know any better. My patients who take brand name get better outcomes. It's not rocket science. If it was that simple, why do you think the brand names cost more? Because they work better. Duh.
Actually I’ve seen way too many patients crash after switching generics. Especially on carbamazepine. One guy had three seizures in two weeks. His doctor said "it’s the same thing"-but it wasn’t. The absorption profile was off. And no one tested him for a week. That’s not science. That’s negligence.
Don’t pretend this is black and white. It’s not. The exceptions exist for a reason. And if you’re not monitoring, you’re not caring.
eh whatever. generics are fine. i don't care. just give me the cheapest one.
Just had a patient come in last week who’d been on brand-name levothyroxine for 10 years. Switched to generic because her insurance dropped the brand. TSH went from 2.1 to 6.8 in six weeks. We switched her back. She’s fine now.
Point is: for some drugs, consistency matters. It’s not about brand vs generic-it’s about stability. If a patient’s been stable on a brand for years? Leave it alone. Don’t fix what isn’t broken.
And yes, I prescribe generic by default for 90% of my scripts. But I don’t treat patients like lab rats. I treat them like people.